Ordering Compliance Guide
Australian law is explicit: medicinal‑cannabis products move only when tied to a valid Therapeutic Goods Administration (TGA) authority. Ignoring this exposes you—and us—to criminal penalties under the Narcotic Drugs Act 1967, the Therapeutic Goods Act 1989, and companion State medicines legislation. Non‑compliant orders are frozen or cancelled without discussion.
The licences behind the rules
- Federal cultivation/manufacture licence (MC038) – authorises production of finished medicinal‑cannabis products.
- Victorian wholesale licences – permit the dispatch of Schedule 3, 4 & 8 cannabis medicines; every movement must be recorded.
- Commonwealth import & export licences – each shipment requires its permit and remains under Canngea control until released against a lawful order.
- Queensland wholesale licence – covers Schedule 3, 4 & 8 cannabinoid products and mandates secure storage, as well as audited record-keeping.
Accordingly, we will not release stock unless the order references one of the following:
- A Special Access Scheme B (SAS‑B) approval number
- An Authorised Prescriber (AP) reference
- A valid CTN/CTA identifier for clinical trials
Exactly how to submit a compliant order
| Visual | Step | Action | Portal Field |
|---|---|---|---|
 |
1 — Open Cart | Click the cart icon when products are selected and confirm every SKU & quantity. | Shopping Cart |
 |
2 — Link Authority to Each Line | Click Add comments for each SKU and paste the item's SAS‑B approval ID or AP reference. No reference = no supply. | Add Comments |
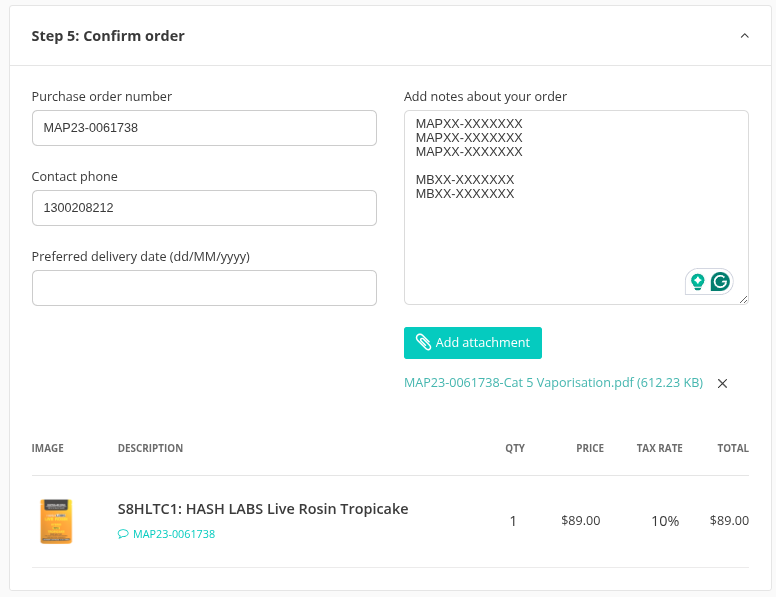 |
3 — Checkout | In the checkout screen: • Enter the primary SAS‑B/AP ref or PO number under Purchase-order number. • Add extra approvals in the Order notes. • Upload all approval letters in the Attachments area. |
Checkout Screen Step 5: Confirm Order |
Real‑time verification: We validate every reference against AP/SASB paperwork immediately. If details are missing or incorrect, the order is placed on hold. Provide correct paperwork within 2 business days, or the order is voided.
Your ongoing obligations
- Maintain control records for three years.
- Store Schedule 8 & 4 products in compliant safes or vaults — once stock leaves our custody, it cannot re‑enter.
- Use approved secure couriers only. Parcel lockers and standard postal services are prohibited.
- Please notify us immediately of any changes to your authority status, as we are required to report discrepancies to regulators.
Need help before you click “Submit”?
Email orders@canngea.com.au or call 1300 208 212 (Mon–Fri, 09:00 – 17:00 AEST).
Do it right the first time. We will not compromise on legal compliance—neither should you.